Understanding Tumor Markers: What They Are and How They’re Used to Diagnose and Monitor Cancer
Tumor markers are valuable tools in cancer care, helping to detect certain cancers early, monitor the effectiveness of treatments, and check for recurrences. However, they are not standalone diagnostic tools and must be interpreted in conjunction with a broader diagnostic workup. By understanding their role, healthcare providers can better manage cancer diagnosis and treatment, providing more precise and effective care for patients.
It’s important to note that not all cancers have associated tumor markers, and not all individuals with cancer will have elevated levels of a particular marker. Tumor markers are most useful when used in conjunction with other diagnostic tests, and their interpretation should always be done in the context of a person’s overall medical history and other diagnostic tests.
Tumor markers play a crucial role in the early detection and monitoring of various cancers, serving as valuable indicators in the diagnostic process and treatment management.
While tumor markers are valuable tools in the detection and management of certain cancers, they are not universal detectors for all cancer types. Tumor markers are specific to particular cancers or even specific subtypes within a cancer type. Moreover, the presence of a tumor marker does not guarantee the presence of cancer, and elevated levels can also be observed in benign conditions or other diseases.
How is the tumor makers done?
Tumor marker tests are blood tests that measure the levels of specific substances produced by cancer cells or normal cells in response to the presence of cancer. These tests are commonly used in cancer management, including cancer screening, diagnosis, monitoring treatment response, and detecting cancer recurrence. The process of performing tumor marker tests typically involves the following steps:
- Blood Sample Collection: The first step is to collect a blood sample from the patient. Blood samples are usually taken from a vein in the arm using a needle and syringe or by using a finger prick in some cases.
- Laboratory Analysis: The blood sample is sent to a laboratory where it undergoes analysis. In the laboratory, the blood is centrifuged to separate the different components, and then the serum or plasma is used for testing.
- Assaying the Tumor Marker: Various laboratory techniques, such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), or chemiluminescent immunoassay (CLIA), are used to measure the concentration of the specific tumor marker in the blood sample.
- Interpretation of Results: Results are reviewed by healthcare providers, but high marker levels require further testing to confirm cancer.
It’s important to note that tumor marker tests are not definitive diagnostic tools for cancer. Elevated levels of a tumor marker can indicate the possibility of cancer, but further diagnostic evaluations, such as imaging tests, biopsies, and clinical assessments, are necessary to confirm a cancer diagnosis.
Tumor Markers to Detect Various Cancers
Tumor markers have emerged as essential tools in the field of oncology, offering valuable insights into the detection, diagnosis, and management of various cancers. These biomolecules, produced by cancer cells or normal cells in response to the presence of cancer, provide clinicians with critical information about the presence and progression of cancer, aiding in early detection, treatment planning, and monitoring of therapeutic response. Through ongoing research and advancements in medical technology, tumor markers continue to pave the way for improved patient outcomes and personalized cancer care strategies. Here are some tumor markers to detect various cancers:
Tumor markers to detect breast cancer
While there are several tumor markers associated with breast cancer, they are not routinely used as standalone tests for breast cancer screening or diagnosis. Instead, they are used in conjunction with other diagnostic methods and clinical evaluation to aid in the management of breast cancer.
Some of the commonly used tumor markers for breast cancer include:
- CA 15-3: CA 15-3 is a glycoprotein that can be elevated in the blood of some patients with breast cancer. It is used to monitor treatment response and disease progression in advanced or metastatic breast cancer.
- CA 27.29: CA 27.29 is another marker used to monitor treatment response and disease progression in advanced breast cancer. It is also used for surveillance in patients with a history of breast cancer.
- HER2/neu (Human Epidermal Growth Factor Receptor 2): HER2 is a protein that plays a role in the growth and spread of some breast cancers. HER2 testing is important in determining treatment options, such as targeted therapies like trastuzumab (Herceptin®).
- Estrogen Receptor (ER) and Progesterone Receptor (PR): These receptors are hormone receptors that are commonly tested in breast cancer biopsy samples. Their presence or absence helps guide treatment decisions, such as hormone therapy.
It’s important to emphasize that tumor markers are not definitive diagnostic tools for breast cancer, and their levels may also be elevated in non-cancerous conditions.
The primary methods for diagnosing breast cancer include:
- Mammography: A screening test that uses X-rays to detect abnormalities in the breast.
- Biopsy: A tissue sample is taken from the breast and examined under a microscope to confirm the presence of cancer.
- Breast MRI or Ultrasound: Additional imaging tests may be used to further evaluate breast abnormalities.
If breast cancer is suspected or diagnosed, a healthcare provider will determine the appropriate treatment plan based on the cancer’s stage, type, and other individual factors. Regular breast self-examination, clinical breast examination, and mammography are essential for early detection and improved outcomes for breast cancer.
Understanding Breast Cancer | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect colorectal cancer (colon cancer)
It’s important to note that tumor markers are not used as standalone tests for colorectal cancer screening or diagnosis. Instead, they are used in conjunction with other diagnostic methods and clinical evaluation to aid in the management and treatment of the disease. Some of the commonly used tumor markers for colorectal cancer include:
- Carcinoembryonic Antigen (CEA): CEA is the most widely used tumor marker for colorectal cancer. It may be elevated in the blood of some patients with colorectal cancer, particularly in advanced stages or if the cancer has spread to other parts of the body. CEA is also used to monitor treatment response and disease progression in colorectal cancer.
- CA 19-9: CA 19-9 is another tumor marker that may be elevated in some cases of colorectal cancer. However, it is not specific to colorectal cancer and can also be elevated in other cancers and non-cancerous conditions.
It’s important to emphasize that tumor markers are not definitive diagnostic tools for colorectal cancer, and their levels may also be elevated in non-cancerous conditions. The primary methods for diagnosing colorectal cancer include:
- Colonoscopy: A procedure that allows a healthcare provider to visualize the inside of the colon and rectum and detect any abnormalities, such as polyps or tumors.
- Biopsy: Tissue samples are taken from any suspicious areas identified during colonoscopy and examined under a microscope to confirm the presence of cancer.
- Imaging tests: X-rays, CT scans, MRI, and PET scans can help determine the extent of the cancer and whether it has spread to other parts of the body.
If colorectal cancer is suspected or diagnosed, a healthcare provider will determine the appropriate treatment plan based on the cancer’s stage, location, and other individual factors. Early detection and timely treatment are essential for improving outcomes for colorectal cancer. For individuals at average risk of colorectal cancer, regular screening with colonoscopy is recommended starting at age 50 or earlier based on individual risk factors. Screening can help detect colorectal cancer at an early stage or even prevent it by removing precancerous polyps during colonoscopy.
Colon Cancer (Colorectal) | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect lung cancer
Tumor markers for lung cancer are generally used in conjunction with other diagnostic methods to aid in the management and treatment of the disease. Some of the commonly used tumor markers for lung cancer include:
- Carcinoembryonic Antigen (CEA): CEA is a tumor marker that may be elevated in the blood of some patients with lung cancer. However, it is not specific to lung cancer and can also be elevated in other cancers and non-cancerous conditions.
- CYFRA 21-1: CYFRA 21-1 is a tumor marker that may be elevated in the blood of some patients with non-small cell lung cancer (NSCLC). It is used to monitor treatment response and disease progression in advanced NSCLC.
- ProGRP (Pro-gastrin-releasing peptide): ProGRP is a tumor marker that is sometimes used to monitor small cell lung cancer (SCLC) and assess treatment response.
- NSE (Neuron-Specific Enolase): NSE is a tumor marker that may be used in certain cases of SCLC to monitor treatment response and disease progression.
It’s important to emphasize that tumor markers are not definitive diagnostic tools for lung cancer, and their levels may also be elevated in non-cancerous conditions. The primary methods for diagnosing lung cancer include:
- Imaging tests: X-rays, CT scans, MRI, and PET scans can help identify lung nodules or masses.
- Biopsy: A tissue sample is taken from the lung and examined under a microscope to confirm the presence of cancer.
- Sputum cytology: A sample of sputum (mucus coughed up from the lungs) is examined for cancer cells.
If lung cancer is suspected or diagnosed, a healthcare provider will determine the appropriate treatment plan based on the cancer’s stage, type, and other individual factors. Early detection and timely treatment are essential for improving outcomes for lung cancer. For individuals at high risk of lung cancer, such as smokers or those with a history of exposure to carcinogens, regular screening with low-dose CT scans may be recommended to detect lung cancer at an early stage when it is more treatable.
Lung Cancer | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect prostate cancer
Tumor markers are substances produced by prostate cancer cells or normal cells in response to the presence of prostate cancer. However, unlike some other cancers, there is no single tumor marker that can definitively diagnose prostate cancer or be used alone for prostate cancer screening. Tumor markers for prostate cancer are generally used in conjunction with other diagnostic methods to aid in the management and treatment of the disease. Some of the commonly used tumor markers for prostate cancer include:
- Prostate-Specific Antigen (PSA): PSA is the most well-known and widely used tumor marker for prostate cancer. It is a protein produced by the prostate gland, and elevated levels of PSA in the blood can be an indicator of prostate cancer. However, PSA levels can also be elevated in non-cancerous conditions, such as benign prostatic hyperplasia (BPH) and prostatitis.
- Prostate Health Index (PHI): PHI is a test that combines PSA, free PSA, and p2PSA (a subtype of PSA) to provide a more accurate assessment of the risk of prostate cancer. It helps distinguish between aggressive and non-aggressive prostate cancer, reducing unnecessary biopsies.
- 4Kscore Test: The 4Kscore Test is another blood test that measures four kallikrein markers (total PSA, free PSA, intact PSA, and human kallikrein 2) to estimate the risk of high-grade prostate cancer.
It’s important to emphasize that tumor markers, including PSA, are not definitive diagnostic tools for prostate cancer, and their levels may also be elevated in non-cancerous conditions. The primary methods for diagnosing prostate cancer include:
- Digital Rectal Exam (DRE): A physical examination of the prostate through the rectum to check for any abnormalities or lumps.
- Transrectal Ultrasound (TRUS): An imaging test that uses sound waves to visualize the prostate and assess its size and shape.
- Prostate Biopsy: Tissue samples are taken from the prostate gland and examined under a microscope to confirm the presence of cancer.
If prostate cancer is suspected or diagnosed, a healthcare provider will determine the appropriate treatment plan based on the cancer’s stage, grade, and other individual factors. Early detection and timely treatment are essential for improving outcomes for prostate cancer. For men at average risk of prostate cancer, regular prostate cancer screening with PSA and DRE is recommended starting at age 50 or earlier based on individual risk factors. Screening can help detect prostate cancer at an early stage when it is more treatable.
Prostate Cancer | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect stomach (gastric) cancer
Tumor markers are substances produced by stomach (gastric) cancer cells or normal cells in response to the presence of stomach cancer. However, it’s important to note that tumor markers are not used as standalone tests for stomach cancer screening or diagnosis. Instead, they are used in conjunction with other diagnostic methods and clinical evaluation to aid in the management and treatment of the disease. Some of the commonly used tumor markers for stomach cancer include:
- Carcinoembryonic Antigen (CEA): CEA is a tumor marker that may be elevated in the blood of some patients with stomach cancer. However, it is not specific to stomach cancer and can also be elevated in other cancers and non-cancerous conditions.
- CA 19-9: CA 19-9 is another tumor marker that may be elevated in some cases of stomach cancer. Similar to CEA, it is not specific to stomach cancer and can be elevated in other gastrointestinal cancers and non-cancerous conditions.
It’s important to emphasize that tumor markers are not definitive diagnostic tools for stomach cancer, and their levels may also be elevated in non-cancerous conditions. The primary methods for diagnosing stomach cancer include:
- Endoscopy: A procedure that allows a healthcare provider to visualize the inside of the stomach and obtain tissue samples (biopsies) for examination under a microscope to confirm the presence of cancer.
- Imaging tests: X-rays, CT scans, MRI, and PET scans can help determine the extent of the cancer and whether it has spread to other parts of the body.
- Stomach Biopsy: Tissue samples are taken from the stomach during endoscopy and examined under a microscope to confirm the presence of cancer.
If stomach cancer is suspected or diagnosed, a healthcare provider will determine the appropriate treatment plan based on the cancer’s stage, location, and other individual factors. Early detection and timely treatment are essential for improving outcomes for stomach cancer. For individuals at increased risk of stomach cancer, such as those with a family history of the disease or certain genetic conditions, regular screening and surveillance may be recommended. Screening can help detect stomach cancer at an early stage when it is more treatable.
Stomach Cancer (Gastric) | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect kidney cancer
For kidney cancer, there are several tumor markers that may be used in the diagnosis, prognosis, and monitoring of the disease. However, it’s important to note that these tumor markers are not specific to kidney cancer and may also be associated with other conditions or cancers. Additionally, tumor markers are not typically used as stand-alone tests for cancer diagnosis but rather as part of a comprehensive evaluation along with imaging studies, biopsies, and other diagnostic tests.
Some of the tumor markers that may be used in kidney cancer include:
- Renal Cell Carcinoma Antigen (RCC-Ag): RCC-Ag is a tumor marker specific to renal cell carcinoma (RCC), the most common type of kidney cancer. It may be used to monitor the progress of the disease and assess treatment response.
- Carbonic Anhydrase IX (CA IX): CA IX is a protein that is overexpressed in the majority of clear cell renal cell carcinomas, the most common subtype of RCC. It can be used as a potential marker for diagnosing and monitoring clear cell RCC.
- Neutrophil Gelatinase-Associated Lipocalin (NGAL): NGAL is a protein that can be elevated in the blood and urine of patients with kidney cancer. It may be used as a biomarker for the early detection of kidney cancer or to monitor disease progression.
- Cystatin C: Cystatin C is a marker of kidney function and may be used to assess kidney health in patients with kidney cancer.
- Alpha-fetoprotein (AFP): AFP is not specific to kidney cancer but may be used in some cases to rule out other types of cancer that can spread to the kidneys.
It’s important to remember that tumor markers are not always elevated in all cases of kidney cancer, and their levels can vary depending on the individual and the specific type and stage of the cancer. Therefore, the interpretation of tumor marker results should be done by a qualified healthcare professional who is familiar with the patient’s medical history, risk factors, and other diagnostic information.
Kidney Cancer | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
Tumor markers to detect Uterine Cancer (Endometrial Cancer)
However, it’s important to note that tumor markers alone are not definitive diagnostic tools for uterine cancer, and they are often used in combination with other diagnostic methods. The most commonly used tumor marker for uterine cancer is:
- CA-125 (Cancer Antigen 125): CA-125 is a protein that can be elevated in the blood of some women with uterine cancer. However, it is not specific to uterine cancer and can also be elevated in other conditions, such as ovarian cancer and certain benign gynecological conditions. Therefore, CA-125 is not used as a standalone test for uterine cancer but can be considered as part of the diagnostic workup.
Other tumor markers, such as HE4 (Human Epididymis Protein 4), may be investigated in research settings, but they are not currently used routinely for uterine cancer detection.
It’s important to emphasize that the primary methods for diagnosing uterine cancer include:
- Transvaginal Ultrasound: This imaging technique allows the visualization of the uterus and can help identify abnormalities, such as thickened endometrial lining or masses.
- Endometrial Biopsy: A sample of the uterine lining is obtained and examined under a microscope to detect cancerous cells or other abnormalities.
- Dilation and Curettage (D&C): A procedure to remove tissue from the lining of the uterus for further examination.
- Imaging Tests: CT scan, MRI, or PET scan may be used to determine the extent of cancer spread.
- Hysteroscopy: A thin, lighted tube (hysteroscope) is used to examine the inside of the uterus for abnormalities.
These diagnostic methods, combined with clinical evaluation and medical history, play a critical role in detecting and diagnosing uterine cancer. If there is a suspicion of uterine cancer based on symptoms or other findings, a healthcare provider may order additional tests, including tumor markers, to aid in the diagnosis and treatment planning. However, a definitive diagnosis is made through the analysis of tissue samples obtained from biopsy or D&C.
Tumor markers to detect Anal Cancer (Anus, Rectal Cancer)
Tumor markers are substances, often proteins, that are produced by cancer cells or by the body in response to cancer. These markers can sometimes be detected in blood, urine, or tissue samples and are used to help diagnose and monitor the progress of certain cancers. While there is no specific tumor marker used exclusively for anal cancer, doctors may use a combination of markers and tests to assess the presence or recurrence of the disease.
1. SCC Antigen (Squamous Cell Carcinoma Antigen)
For anal cancers originating from squamous cells (the most common type of anal cancer), the SCC antigen may be monitored. Elevated levels of SCC antigen in the blood can sometimes indicate the presence of squamous cell carcinoma or signal a recurrence of the cancer. However, elevated SCC antigen levels can also be seen in other squamous cell cancers, such as those in the head and neck or cervix.
2. CEA (Carcinoembryonic Antigen)
CEA is a protein that may be present at higher levels in the blood of patients with certain cancers, including gastrointestinal cancers. Although not specific to anal cancer, CEA may be monitored in some cases, particularly if the cancer has spread or if there is concern about recurrence. Elevated CEA levels can indicate active disease or metastasis in gastrointestinal-related cancers, including adenocarcinoma of the anus (though this is a rare type).
3. HIV and HPV Status
Although not a traditional tumor marker, testing for HIV and HPV status plays a crucial role in the diagnosis and monitoring of anal cancer, especially since HPV infection is a major risk factor. HPV-positive anal cancer has been associated with a better prognosis compared to HPV-negative cancers, and thus monitoring HPV DNA in patients may help assess the risk of recurrence.
Patients with HIV may require additional monitoring because their weakened immune systems can affect the course of the disease and response to treatment. The CD4 count (a measure of immune function in HIV-positive patients) is often monitored, as lower CD4 counts can be associated with a higher risk of cancer progression or recurrence.
Tumor Markers for Womb (Uterine) Cancer
Womb cancer (also known as uterine cancer or endometrial cancer) is primarily detected through imaging and biopsies, but certain tumor markers can also help in diagnosis, monitoring treatment response, and detecting recurrence. Below are the main tumor markers associated with uterine cancer:
1. CA-125 (Cancer Antigen 125)
CA-125 is the most commonly used tumor marker for detecting uterine cancer, especially in advanced stages or in cases of endometrial cancer that has spread beyond the uterus. While elevated CA-125 levels are not specific to uterine cancer (they can be raised in ovarian, cervical, or other cancers), they can provide valuable information about the disease’s progression and response to treatment. This marker is often monitored during follow-up care to detect recurrence.
2. HE4 (Human Epididymis Protein 4)
HE4 is another marker, used alongside CA-125, particularly in the context of distinguishing uterine cancer from ovarian cancer. HE4 levels are often evaluated in women with endometrial cancer to assess the likelihood of cancer spread and recurrence.
3. CEA (Carcinoembryonic Antigen)
While CEA is commonly used in gastrointestinal cancers, it can also be elevated in certain cases of uterine cancer, particularly when there is metastasis or involvement of other organs. CEA may be measured in advanced or recurrent cases.
4. AFP (Alpha-Fetoprotein)
In rare cases of uterine sarcoma or uterine carcinosarcoma, AFP may be elevated. However, this marker is not typically used in standard endometrial cancer diagnosis but can be monitored for rarer types of uterine cancers.
Monitoring and Detection
- CA-125 and HE4 levels are commonly monitored after initial treatment to detect recurrence.
- These markers are used in combination with imaging (CT, MRI) and physical exams to provide a comprehensive assessment of the cancer’s status.
Common Name of Tumor Markers Tests: Uses, Limitations, and What They Mean for Cancer Diagnosis and Management
Here is a list of some common tumor markers, along with their uses and limitations:
Carcinoembryonic antigen (CEA): tumor marker associated with various types of cancers, most notably colorectal cancer and other gastrointestinal cancers.
CEA is a marker commonly used for colon cancer and rectal cancer. It can also be elevated in other types of cancer, including lung, breast, pancreatic, and ovarian cancer. However, CEA can also be elevated in non-cancerous conditions such as inflammatory bowel disease, smoking, and liver disease.
CEA testing is often used as a tumor marker to help detect and monitor the progression of these types of cancer. However, CEA levels can also be elevated in non-cancerous conditions, such as inflammation, infection, and liver disease. Therefore, CEA testing is not a definitive test for cancer and must be used in conjunction with other diagnostic tests, such as imaging and biopsies.
CEA testing may also be used to monitor the effectiveness of cancer treatment and detect cancer recurrence. However, like other tumor markers, CEA testing has limitations and can lead to false positives and false negatives.
The cancers that CEA is used to detect and monitor includes:
- Colorectal Cancer: CEA is most commonly used as a tumor marker for colorectal cancer. It can be helpful in assessing treatment response and monitoring for cancer recurrence after surgery or other treatments.
- Other Gastrointestinal Cancers: CEA levels may also be elevated in other gastrointestinal cancers, such as pancreatic cancer, gastric (stomach) cancer, and liver cancer (hepatocellular carcinoma).
- Other Cancers: In addition to gastrointestinal cancers, CEA levels can be elevated in some cases of lung cancer, breast cancer, and certain tumors of the ovaries, uterus, and thyroid.
Alpha-fetoprotein (AFP): Tumor markers for the following cancers: liver, testicular, ovarian, biliary tract, gastric or stomach
AFP is a marker used for liver cancer, testicular cancer and ovarian (germ cell tumors), biliary tract cancer, gastric or stomach cancer and ovarian cancer. It is also elevated in pregnant women, and in infants with certain birth defects.
Alpha-fetoprotein (AFP) is a protein that is normally produced during fetal development and is found in high levels in the blood of developing fetuses. However, after birth, AFP levels decrease rapidly and are typically very low in healthy adults.
AFP testing may also be used to monitor the effectiveness of cancer treatment and detect cancer recurrence. However, like other tumor markers, AFP testing has limitations and can lead to false positives and false negatives.
The cancers commonly associated with elevated AFP levels include:
- Liver Cancer (Hepatocellular Carcinoma – HCC): AFP is most commonly used as a tumor marker for liver cancer. Elevated AFP levels are found in a significant number of people with hepatocellular carcinoma, especially in cases of more advanced disease.
- Germ Cell Tumors: AFP is also associated with certain germ cell tumors, including:
a. Testicular Cancer: Non-seminomatous testicular cancers, such as embryonal carcinoma, yolk sac tumor, and teratoma, can produce AFP.
b. Ovarian Cancer: Germ cell tumors of the ovary, particularly yolk sac tumors, can also lead to increased AFP levels.
- Biliary Tract Cancer: Some cases of biliary tract cancer, such as cholangiocarcinoma, may produce AFP, although this is relatively rare.
- Gastric (stomach) Cancer: In a small percentage of cases of gastric cancer (stomach cancer), elevated AFP levels may be observed.
It is crucial to remember that AFP levels can also be elevated in certain non-cancerous conditions, such as liver diseases, hepatitis, and cirrhosis. Therefore, the interpretation of AFP levels should always be done in conjunction with other diagnostic tests and medical evaluation by a healthcare professional.
Regular monitoring of AFP levels is essential for individuals with a history of cancers associated with AFP elevation or those undergoing cancer treatment to track the progress and response to therapy. Early detection and timely treatment play a critical role in improving the outcomes for individuals with these types of cancers.
http://cleverlysmart.com/afp-alpha-fetoprotein-tumor-marker-blood-test/
Prostate-specific antigen (PSA): associated with prostate cancer
PSA is a marker used for prostate cancer. However, PSA can also be elevated in non-cancerous conditions such as prostate infection and benign prostatic hyperplasia (BPH).
Elevated levels of PSA can be a sign of prostate cancer, but they can also be caused by other non-cancerous conditions, such as an enlarged prostate or an infection. Therefore, PSA testing is not a definitive test for prostate cancer, and further testing, such as a biopsy, may be needed to confirm the diagnosis.
PSA testing is controversial because it can lead to overdiagnosis and overtreatment of prostate cancer, particularly in men who have low-grade, slow-growing tumors that may not require treatment. However, PSA testing can also be valuable in detecting aggressive forms of prostate cancer early, when they are more treatable.
Men should discuss the risks and benefits of PSA testing with their healthcare provider, taking into account their age, family history, and other risk factors for prostate cancer.
Prostate Cancer | Symptoms, Stages, Types, Diagnoses, Chances of Surviving, Treatments
PSA density (PSAD): is not a tumor marker for specific cancers. Instead, it is a calculated value used in conjunction with the prostate-specific antigen (PSA) test to assess the risk of prostate cancer in individuals.
This is a marker used to assess the risk of prostate cancer in men with elevated PSA levels. PSAD is calculated by dividing the PSA level by the size of the prostate gland as measured on ultrasound.
PSAD takes into account both the PSA level and the size of the prostate, providing a more accurate assessment of prostate cancer risk. A higher PSAD may indicate a higher risk of prostate cancer, particularly if the PSA level is also elevated.
PSAD is often used in conjunction with other diagnostic tests, such as a digital rectal exam (DRE) or a prostate biopsy, to help confirm the diagnosis of prostate cancer and determine the best course of treatment. However, like PSA testing, PSAD has limitations and can lead to overdiagnosis and overtreatment of low-grade, slow-growing tumors.
PSAD is a measurement that takes into account the PSA level and the size of the prostate gland. It is calculated by dividing the PSA value by the volume of the prostate, usually determined through imaging studies like transrectal ultrasound (TRUS) or magnetic resonance imaging (MRI). The formula for PSAD is:
PSAD = PSA level (ng/mL) / Prostate volume (mL)
PSAD is not used to detect or diagnose cancer by itself, but rather it helps in refining the interpretation of PSA results in individuals with an enlarged prostate gland (BPH) or other prostate conditions. The reason for using PSAD is that PSA levels can be influenced by the size of the prostate, and larger prostates may naturally produce higher PSA levels even without cancer.
Human kallikrein 2 (hK2): is a tumor marker primarily associated with prostate cancer
Human kallikrein 2 (hK2) is a protein that is produced by the prostate gland.
hK2 is a marker used for prostate cancer. It can also be elevated in non-cancerous conditions such as benign prostatic hyperplasia (BPH). hK2 is used to monitor treatment and detect recurrence.
hK2 testing may also be used to distinguish between benign prostate conditions and prostate cancer, as well as to monitor the effectiveness of prostate cancer treatment and detect cancer recurrence. However, like other tumor markers, hK2 testing has limitations and can lead to false positives and false negatives.
Cancer antigen CA 125: tumor markers associated with ovarian cancer
Cancer antigen CA 125 is a protein that is produced by certain types of cancer cells, including ovarian, endometrial, fallopian tube, and peritoneal cancers.
CA-125 is a marker used for ovarian cancer. It can also be elevated in other types of cancer such as breast, lung, and pancreatic cancer. However, CA-125 can also be elevated in non-cancerous conditions such as menstruation, endometriosis, and liver disease. CA 125 is used to monitor treatment and detect recurrence.
However, not all women with these types of cancers have elevated CA 125 levels, and some women without cancer may have elevated levels of CA 125 due to other conditions, such as endometriosis or pelvic inflammatory disease. Therefore, CA 125 testing is most useful in conjunction with other diagnostic tests and as a tool for monitoring cancer treatment and detecting cancer recurrence.
CA 125 testing is not recommended as a screening test for ovarian or other types of cancer in the general population, as it may lead to unnecessary testing and treatment in individuals who do not have cancer.
Human chorionic gonadotropin (HCG): tumor marker associated with certain types of cancers, most notably germ cell tumors and trophoblastic tumors. hCG is a hormone produced by the placenta during pregnancy, but it can also be produced by certain cancer cells.
HCG is a marker used for germ cell tumors such as testicular cancer and ovarian cancer. It is also elevated in pregnant women. It can also be elevated in non-cancerous conditions such as pregnancy and some types of pituitary gland disorders. hCG is used to monitor treatment and detect recurrence.
Human chorionic gonadotropin (HCG) is a hormone that is produced by the placenta during pregnancy. Elevated levels of HCG in the blood or urine can be a sign of pregnancy and may be used as a diagnostic test to confirm pregnancy.
However, HCG can also be produced by certain types of cancer cells, such as testicular, ovarian, or germ cell tumors. Therefore, HCG testing may also be used as a tumor marker to help detect and monitor the progression of these cancers.
HCG testing can also be used to diagnose and monitor certain non-cancerous conditions, such as trophoblastic disease or pituitary gland disorders. However, like other tumor markers, HCG testing has limitations and can lead to false positives and false negatives.
The cancers that hCG is used to detect and monitor include:
- Germ Cell Tumors:
- Testicular Cancer: Non-seminomatous testicular cancers, such as choriocarcinoma and embryonal carcinoma, can produce hCG.
- Ovarian Cancer: Germ cell tumors of the ovary, particularly choriocarcinoma, can also lead to increased hCG levels.
- Extragonadal Germ Cell Tumors: These are germ cell tumors that originate outside the gonads, and they can produce hCG.
- Testicular Cancer: Non-seminomatous testicular cancers, such as choriocarcinoma and embryonal carcinoma, can produce hCG.
- Trophoblastic Tumors:
Gestational Trophoblastic Disease (GTD): Trophoblastic tumors arise from abnormal growth of placental tissue and can include conditions like hydatidiform mole, choriocarcinoma, and placental-site trophoblastic tumors. hCG is a critical marker in the diagnosis and monitoring of GTD.
Overall, HCG testing is most useful in conjunction with other diagnostic tests and as a tool for monitoring cancer treatment and detecting cancer recurrence. It is not recommended as a screening test for cancer in the general population.

The structure of Human chorionic gonadotropin (hCG). Borislav Mitev – borislav_mitev@hotmail.com, Public domain, via Wikimedia Commons
hCG beta total
The hCG beta total is a blood test that measures the total amount of beta subunit hCG in the bloodstream. This test is used to confirm pregnancy and to monitor the progression of pregnancy. It can also be used to detect certain types of cancer, including testicular and ovarian cancer, which can produce hCG.
There are two main forms of hCG: alpha and beta. Beta-hCG is the most specific form of the hormone for detecting pregnancy and is the form that is typically measured in blood or urine tests. In addition to its use in pregnancy tests, beta-hCG levels can also be used to monitor the progression of pregnancy and to diagnose certain types of cancer.
Alpha hCG
Alpha-hCG, also known as the alpha subunit of human chorionic gonadotropin, is a protein that is produced by the placenta during pregnancy. Alpha-hCG is not specific to pregnancy, as it is also produced by certain types of cancer, including testicular and ovarian cancer.
While measuring alpha-hCG levels alone is not typically used to diagnose pregnancy, it can be useful in certain cases where beta-hCG levels are not detectable or are unreliable, such as in ectopic pregnancies or in cases of trophoblastic disease (refers to the tissue that makes up the outer layer of the blastocyst in early pregnancy, which is responsible for implanting the embryo and forming the placenta. It can also refer to abnormal growth of this tissue in certain types of tumors, which can produce pregnancy-related hormones such as hCG.).
Alpha-hCG levels can also be used in conjunction with beta-hCG levels to monitor the progression of pregnancy, as both alpha and beta subunits are produced by the placenta and their levels increase throughout the course of a healthy pregnancy. In cases of cancer, elevated levels of alpha-hCG may indicate the presence of certain types of tumors, such as testicular or ovarian cancer.
Human chorionic gonadotropin beta (hCG beta)
hCG beta is a marker used for some types of testicular cancer and gestational trophoblastic disease. It can also be elevated in non-cancerous conditions such as pregnancy and some types of pituitary gland disorders.
Measuring the levels of human chorionic gonadotropin beta (hCG beta) in the blood can be used as a diagnostic tool for certain types of cancer, including testicular and ovarian cancer. In these types of cancer, hCG beta is often produced by the tumor cells and can be detected in the blood.
In testicular cancer, hCG beta is produced by certain types of tumor cells, and measuring its levels can be used to monitor the response to treatment and detect any recurrence of the cancer. In addition to hCG beta, alpha-fetoprotein (AFP) and lactate dehydrogenase (LDH) are also commonly measured in the blood to monitor testicular cancer.
In ovarian cancer, hCG beta is produced by some types of tumors, and its levels can be measured to help diagnose the cancer and monitor the response to treatment. However, hCG beta is not a specific marker for ovarian cancer, and its levels may also be elevated in pregnancy or in other types of cancers.
It is important to note that while measuring hCG beta levels can be a useful tool in diagnosing and monitoring certain types of cancer, it should always be used in conjunction with other diagnostic tests and clinical assessments.
CA 19-9: tumor markers associated with certain types of gastrointestinal cancers, most notably pancreatic cancer.
CA 19-9 is a protein that is produced by certain types of cancer cells, including pancreatic, biliary tract, and gastric cancers.
CA 19-9 is a tumor marker used for pancreatic cancer and sometimes for other types of cancer such as colorectal cancer and other gastrointestinal cancers. However, CA 19-9 can also be elevated in non-cancerous conditions such as pancreatitis and liver disease. CA 19-9 is used to monitor treatment and detect recurrence.
The cancers that CA 19-9 is used to detect and monitor include:
- Pancreatic Cancer: CA 19-9 is most commonly used as a tumor marker for pancreatic cancer. It can be helpful in assessing the extent of the disease, monitoring treatment response, and detecting cancer recurrence after surgery or other treatments.
- Gastrointestinal Cancers: In addition to pancreatic cancer, CA 19-9 levels may also be elevated in other gastrointestinal cancers, including bile duct cancer (cholangiocarcinoma) and gastric (stomach) cancer.
- Other Cancers: CA 19-9 levels can also be elevated in some cases of bile duct obstruction, liver disease, and other non-cancerous conditions. Therefore, CA 19-9 testing is not specific to cancer and should not be used as a stand-alone test to diagnose cancer.
However, not all individuals with these types of cancers have elevated CA 19-9 levels, and some individuals without cancer may have elevated levels of CA 19-9 due to other conditions, such as pancreatitis or liver disease. Therefore, CA 19-9 testing is most useful in conjunction with other diagnostic tests and as a tool for monitoring cancer treatment and detecting cancer recurrence.
CA 19-9 testing is not recommended as a screening test for pancreatic or other types of cancer in the general population, as it may lead to unnecessary testing and treatment in individuals who do not have cancer. Additionally, the use of CA 19-9 testing as a diagnostic tool for pancreatic cancer is limited, as the test may not be elevated in early stages of the disease.
CA 15-3: is a tumor marker primarily associated with breast cancer
CA 15-3 is a marker used for breast cancer. However, it can also be elevated in other types of cancer, such as ovarian and lung cancer. CA 15 3 can also be elevated in non-cancerous conditions, such as liver disease and breast cysts. Elevated CA 15-3 levels can indicate a more aggressive form of breast cancer, and it is used to monitor treatment and detect recurrence.
CA 15-3 testing is most useful in conjunction with other diagnostic tests, such as mammograms and biopsies, to aid in the diagnosis of breast cancer and to monitor treatment progress. It is not recommended as a screening test for breast cancer in the general population, as it is not sensitive or specific enough to be used as a standalone diagnostic tool.
Additionally, while CA 15-3 levels may be useful in monitoring breast cancer treatment, it should not be the sole indicator of cancer recurrence or progression. Other imaging tests, such as mammograms and PET scans, may be used in conjunction with CA 15-3 testing to provide a more accurate assessment of cancer progression.
CA 27.29: tumor marker primarily associated with breast cancer.
It is a specific form of the cancer antigen 15-3 (CA 15-3) and is used to monitor and assess breast cancer progression and treatment response.
CA 27.29 is a marker used for breast cancer. It is typically used to monitor treatment and detect cancer recurrence in people with advanced breast cancer. However, it can also be elevated in other types of cancer, such as ovarian and lung cancer.
This is a protein that is produced by some breast cancer cells. It can be measured in the blood as a tumor marker to help detect and monitor the progression of breast cancer. Similar to CA 15-3, CA 27.29 testing is most useful in conjunction with other diagnostic tests, such as mammograms and biopsies, to aid in the diagnosis of breast cancer and to monitor treatment progress.
However, it’s important to note that not all individuals with breast cancer have elevated CA 27.29 levels, and some individuals without breast cancer may have elevated levels of CA 27.29 due to other conditions, such as liver disease. Additionally, like other tumor markers, CA 27.29 should not be used as a standalone diagnostic tool and should be interpreted in the context of a patient’s clinical history and other diagnostic test results.
Overall, CA 27.29 can be a useful tool for monitoring breast cancer treatment and detecting cancer recurrence, but it is not a definitive diagnostic test and should be used in combination with other tests for the most accurate assessment of breast cancer status.
Human epididymis protein 4 (HE4): is a tumor marker associated with certain gynecological cancers, most notably ovarian cancer.
HE4 is a marker used for ovarian cancer, particularly for monitoring treatment and detecting recurrence. HE4 is less likely to be elevated in non-cancerous conditions than CA-125. It can also be elevated in non-cancerous conditions such as endometriosis and pelvic inflammatory disease. HE4 is used to monitor treatment and detect recurrence.
Testing for HE4 is done using a blood test, which measures the level of HE4 in the blood. HE4 is often used in combination with another tumor marker, CA-125, to improve the accuracy of ovarian cancer detection. While CA-125 is more commonly used, it can be elevated in other conditions besides ovarian cancer, so HE4 can provide additional information for more accurate diagnosis.
HE4 testing can be particularly useful in monitoring the progress of ovarian cancer treatment, as changes in HE4 levels over time can indicate response to therapy. In addition, some studies have shown that HE4 may be a better predictor of ovarian cancer in certain populations, such as premenopausal women, compared to CA-125.
Active Cancer Surveillance and Visits | Monitoring and Follow-up for Cancer Survivors
Beta-2 microglobulin (B2M): is a tumor marker associated with certain types of blood-related cancers, particularly multiple myeloma and some lymphomas.
B2M is a marker used for some types of blood cancer, such as multiple myeloma and lymphoma. It can also be elevated in kidney disease and autoimmune disorders. Elevated B2M levels can also be seen in non-cancerous conditions such as rheumatoid arthritis and kidney disease.
The cancers that B2M is used to detect and monitor include:
- Multiple Myeloma: B2M is commonly used as a tumor marker for multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. Elevated B2M levels can indicate the presence and progression of multiple myeloma.
- Some Lymphomas: In some cases of certain types of lymphomas, particularly those that involve B-cells, B2M levels may be elevated.
In medicine, B2M is most commonly used as a marker of kidney function, as it is filtered by the kidneys and excreted in the urine. Elevated levels of B2M in the blood or urine can indicate impaired kidney function.
B2M is also used as a tumor marker for some types of cancer, such as multiple myeloma and some lymphomas. In these cancers, B2M is produced in higher amounts by the cancer cells and can be detected in the blood. Monitoring B2M levels can help doctors assess the effectiveness of treatment and detect disease progression.
In addition to its use as a marker for kidney function and cancer, B2M has also been associated with other conditions, such as autoimmune disorders and HIV infection. Elevated levels of B2M in the blood have been linked to an increased risk of death in people with HIV.
Neuron-specific enolase (NSE): is a tumor marker associated with certain types of neuroendocrine tumors, particularly those originating from neuroendocrine cells.
NSE is a marker used for certain types of neuroendocrine tumors, such as small cell lung cancer and neuroblastoma. It can also be elevated in other types of cancer, such as thyroid cancer and pancreatic cancer. NSE levels can also be elevated in non-cancerous conditions, such as stroke and seizures.
In medicine, NSE is most commonly used as a tumor marker for certain types of cancer, particularly neuroendocrine tumors such as small cell lung cancer and neuroblastoma. In these cancers, NSE is produced in higher amounts by the cancer cells and can be detected in the blood. Monitoring NSE levels can help doctors assess the effectiveness of treatment and detect disease progression.
NSE is also used as a marker of neurological injury, particularly in the context of traumatic brain injury, stroke, and hypoxic-ischemic encephalopathy (a type of brain injury that occurs when the brain doesn’t receive enough oxygen). Elevated levels of NSE in the blood can indicate damage to neurons and can help doctors assess the severity of the injury and predict outcomes.
The cancers that NSE is used to detect and monitor include:
- Neuroendocrine Tumors: NSE is most commonly used as a tumor marker for neuroendocrine tumors, which can arise in various organs, including the lungs, gastrointestinal tract, pancreas, and other parts of the body. Neuroendocrine tumors are a diverse group of cancers that produce hormones and other substances, and they can have varying degrees of aggressiveness.
- Small Cell Lung Cancer (SCLC): NSE is particularly useful in the diagnosis and monitoring of small cell lung cancer, a type of lung cancer that is often associated with neuroendocrine features.
Alpha-1-fetoprotein (AFP-L3): is a specific form of alpha-1-fetoprotein (AFP) and is a tumor marker associated with certain types of liver cancers, particularly hepatocellular carcinoma (HCC). AFP-L3 testing is used to detect and monitor HCC, which is the most common type of primary liver cancer.
AFP-L3 is a subtype of AFP that is used to monitor treatment and detect recurrence of liver cancer. It is more specific to liver cancer than the regular AFP marker. It is a subtype of AFP that has a particular structure that allows it to be distinguished from other forms of AFP. AFP is normally produced in the liver and yolk sac of a developing fetus and is found in the blood of pregnant women and in the blood of people with certain diseases, including liver cancer.
AFP-L3 is a more specific marker for liver cancer than total AFP because it is produced in higher amounts by liver cancer cells than by normal liver cells. AFP-L3 is typically measured as a percentage of total AFP in the blood, and a high percentage of AFP-L3 in relation to total AFP is considered to be a strong indicator of the presence of liver cancer.
AFP-L3 is often used in combination with other tumor markers and imaging tests to diagnose and monitor liver cancer. However, it is important to note that a high level of AFP-L3 does not definitively indicate the presence of liver cancer, and additional testing is typically needed to confirm a diagnosis.
Human epidermal growth factor receptor 2 (HER2): is a tumor marker associated with breast cancer.
HER2 is a marker used for breast cancer. It is a protein that is found on the surface of some breast cancer cells. Elevated levels of HER2 can indicate a more aggressive form of breast cancer, and targeted therapies are available to treat HER2-positive breast cancer.
HER2-positive breast cancer occurs when the cancer cells have an excess of HER2 receptors on their surface. This excess of receptors can cause the cancer cells to grow and divide more rapidly, and is associated with a more aggressive form of the disease.
HER2 testing is an important part of breast cancer diagnosis and treatment planning. It is typically done using a tissue sample from a breast biopsy or surgery. HER2 status is determined by measuring the level of HER2 protein expression or the presence of HER2 gene amplification in the tissue sample. HER2-positive breast cancer can be treated with targeted therapies such as trastuzumab (Herceptin), which binds to the HER2 receptor and inhibits its activity, slowing or stopping the growth of the cancer cells.
In addition to breast cancer, HER2 overexpression has also been found in other types of cancer, including some types of gastric, ovarian, and lung cancer. HER2 testing and targeted therapies are also being investigated for these cancers.
KRAS mutation: is a gene that can undergo mutations in certain cancers, particularly colorectal cancer and some other types of cancers. KRAS mutation testing is not a tumor marker in the traditional sense, but it is a genetic test used to identify specific mutations in the KRAS gene that can affect cancer development and treatment response.
KRAS (Kirsten rat sarcoma viral oncogene homolog) is a gene that can be mutated in several different types of cancer, such as lung cancer, colorectal cancer, and pancreatic cancer. KRAS mutations can be detected through genetic testing of tumor tissue, and targeted therapies are available for some KRAS-mutated cancers.
This is is a gene that provides instructions for making a protein that is involved in cell signaling pathways that control cell growth and division. When mutations occur in the KRAS gene, the protein that is produced may become permanently activated, leading to uncontrolled cell growth and division.
Testing for KRAS mutations can help guide treatment decisions, as certain targeted therapies have been developed that can inhibit the activity of mutated KRAS proteins.
It is important to note that not all KRAS mutations are the same, and some may be more susceptible to targeted therapies than others. Therefore, additional testing may be needed to determine the specific type of KRAS mutation present and the most appropriate treatment approach.
BRAF mutation: is a gene that can undergo mutations in certain cancers. BRAF mutation testing is not a tumor marker in the traditional sense, but it is a genetic test used to identify specific mutations in the BRAF gene that can affect cancer development and treatment response.
The BRAF (v-Raf murine sarcoma viral oncogene homolog B1) mutation is a genetic alteration in the BRAF gene that has been linked to several types of cancer, including melanoma, colorectal cancer, and thyroid cancer. The most common type of BRAF mutation is the V600E mutation, which accounts for about 90% of all BRAF mutations.
The cancers that BRAF mutation testing is used to detect and monitor include:
- Melanoma: BRAF mutations are commonly found in a significant proportion of melanomas, particularly in cutaneous melanoma, which is the most common form of skin cancer. The most common BRAF mutation in melanoma is the V600E mutation.
- Colorectal Cancer: BRAF mutations can also occur in a subset of colorectal cancers, particularly those that have a specific type of genetic alteration known as microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR).
- Other Cancers: BRAF mutations are less common but can also be found in other types of cancers, such as papillary thyroid cancer, hairy cell leukemia, and some types of ovarian and lung cancers.
The BRAF gene provides instructions for making a protein that helps regulate cell growth and division. When a mutation occurs in the BRAF gene, it can cause the protein to become overactive, leading to uncontrolled cell growth and the development of cancer.
Testing for the BRAF mutation can be done through a biopsy or blood test. Knowing whether a patient has the mutation can help doctors determine the best course of treatment. For example, some drugs have been developed specifically to target cancer cells with the BRAF mutation.
It’s important to note that not all cancer patients have the BRAF mutation, and having the mutation does not necessarily mean that a person will develop cancer. Additionally, not all targeted therapies are effective for all patients with the mutation.
EGFR mutation: is a mutation testing is used to identify specific mutations in the EGFR gene, which can be found in certain types of cancers, most notably non-small cell lung cancer (NSCLC). EGFR mutations are alterations in the EGFR gene that can affect cancer development and treatment response.
EGFR (Epidermal Growth Factor Receptor) is a gene that can be mutated in certain types of lung cancer. Testing for EGFR mutations can help guide treatment decisions, as some targeted therapies are available for EGFR-mutated lung cancer.
The EGFR mutation refers to a genetic alteration in the epidermal growth factor receptor (EGFR) gene. EGFR is a protein that is involved in cell signaling pathways that control cell growth and division. When mutations occur in the EGFR gene, the protein that is produced may become permanently activated, leading to uncontrolled cell growth and division.
EGFR mutations are particularly common in certain types of cancer, including non-small cell lung cancer, colorectal cancer, and pancreatic cancer. Testing for EGFR mutations can help guide treatment decisions, as certain targeted therapies have been developed that can inhibit the activity of mutated EGFR proteins.
It is important to note that not all EGFR mutations are the same, and some may be more susceptible to targeted therapies than others. Therefore, additional testing may be needed to determine the specific type of EGFR mutation present and the most appropriate treatment approach. Additionally, as with any cancer treatment, the effectiveness of targeted therapies can vary depending on the individual patient and their specific cancer characteristics.
Mucin 1 (MUC1): tumor marker associated with various types of cancers, particularly adenocarcinomas (such as: breast, pancreatic, lung, ovarian, and other cancers.
MUC1 is a marker used for several types of cancer, including breast cancer, ovarian cancer, and pancreatic cancer. Elevated MUC1 levels can indicate a more aggressive form of cancer and can also be used to monitor treatment and detect recurrence.
Mucin 1 (MUC1) is a protein that is found on the surface of many types of cells, including glandular epithelial cells, which are found in the breast, pancreas, and other organs. MUC1 is involved in many cellular processes, including cell signaling, cell adhesion, and cell differentiation.
In some types of cancer, including breast cancer and pancreatic cancer, MUC1 is overexpressed or altered in ways that can promote cancer growth and spread. Therefore, MUC1 has been identified as a potential target for cancer therapies.
The cancers that MUC1 is used to detect and monitor include:
- Breast Cancer: MUC1 is commonly overexpressed in breast cancer cells, especially in more aggressive and advanced forms of the disease. MUC1 testing can aid in the diagnosis and management of breast cancer.
- Pancreatic Cancer: MUC1 is often overexpressed in pancreatic cancer cells and is associated with a poorer prognosis. MUC1 testing may be used to support the diagnosis and treatment decisions for pancreatic cancer.
- Lung Cancer: MUC1 is expressed in some lung cancers, particularly adenocarcinomas. It can be used as a potential marker for lung cancer diagnosis and monitoring.
- Ovarian Cancer: MUC1 is also found to be overexpressed in some ovarian cancers, and its measurement may be used in the management of the disease.
- Other Cancers: MUC1 overexpression has been observed in other cancers, including colon cancer, stomach cancer, and certain types of head and neck cancers.
Researchers are currently investigating various approaches to targeting MUC1 in cancer treatment, including monoclonal antibodies and vaccines that can trigger an immune response against cancer cells that express MUC1. While these approaches show promise, more research is needed to determine their safety and effectiveness in treating cancer.
S-100 protein: is a tumor marker associated with certain types of cancers, particularly those that originate from cells of neural crest origin or melanocytes.
S-100 is a marker used for melanoma and other types of skin cancer. It can also be elevated in non-cancerous conditions such as nerve damage and autoimmune disorders. S-100 is used to monitor treatment and detect recurrence.
The S-100 protein is a type of protein that is found in certain cells of the body, including nerve cells (neurons) and certain types of glial cells in the central and peripheral nervous system. It is a member of the S-100 family of calcium-binding proteins, which play important roles in regulating cell growth, differentiation, and function.
In medicine, S-100 protein is used as a marker for certain types of tumors, particularly those that arise from cells of neural origin, such as melanoma, schwannoma, and astrocytoma. S-100 protein can be detected in the blood or cerebrospinal fluid, and its levels can be measured to monitor the progression of these tumors and assess the response to treatment.
S-100 protein can also be used as a diagnostic tool in certain neurological disorders, such as multiple sclerosis and Alzheimer’s disease. In these conditions, S-100 protein may be elevated in the cerebrospinal fluid, indicating damage to nerve cells or inflammation in the central nervous system.
The cancers that S-100 protein is used to detect and monitor include:
- Melanoma: S-100 protein is commonly used as a tumor marker for melanoma, a type of skin cancer that arises from melanocytes. Melanoma can be aggressive, and S-100 testing can aid in its diagnosis and management.
- Neuroblastoma: S-100 protein can also be elevated in neuroblastoma, a type of cancer that arises from neural crest cells in young children. S-100 testing may be used to support the diagnosis and monitoring of neuroblastoma.
- Schwannoma and Neurofibroma: S-100 protein is expressed in tumors arising from Schwann cells, such as schwannomas and neurofibromas. S-100 testing can help in their diagnosis and differentiation from other tumors.
- Other Cancers: S-100 protein may also be used in certain situations to detect and monitor other cancers, such as malignant peripheral nerve sheath tumors and some types of breast cancer.
Overall, S-100 protein is a useful marker for a variety of medical conditions, particularly those involving the nervous system or tumors of neural origin.
Thyroglobulin (Tg): is a tumor marker associated with thyroid cancer.
Thyroglobulin is a marker used for thyroid cancer. It can also be elevated in non-cancerous conditions such as thyroiditis and goiter. Thyroglobulin is used to monitor treatment and detect recurrence.
The thyroglobulin is a protein that is produced by the thyroid gland, a small butterfly-shaped gland located in the neck. Thyroglobulin is an important component in the synthesis of thyroid hormones, which are essential for regulating metabolism and other functions in the body.
Measuring thyroglobulin levels in the blood is useful in monitoring the health of the thyroid gland and in diagnosing and managing thyroid cancer. After a patient has had their thyroid gland removed due to cancer, their thyroglobulin levels can be monitored as a way to check for any recurrence of the cancer. This is because normal thyroid tissue does not produce significant amounts of thyroglobulin, but thyroid cancer cells may continue to produce it even after the thyroid gland has been removed.
The cancers that thyroglobulin is used to detect and monitor include:
- Differentiated Thyroid Cancer: Thyroglobulin is most commonly used as a tumor marker for differentiated thyroid cancer, which includes papillary thyroid cancer and follicular thyroid cancer. These types of thyroid cancer originate from the follicular cells of the thyroid gland.
- Medullary Thyroid Cancer (MTC): In some cases, thyroglobulin levels may also be elevated in medullary thyroid cancer, a less common type of thyroid cancer that arises from the parafollicular C cells of the thyroid gland.
In addition, measuring thyroglobulin levels in the blood can help determine the effectiveness of thyroid cancer treatment, such as radioactive iodine therapy, which is used to destroy any remaining thyroid cancer cells after surgery.
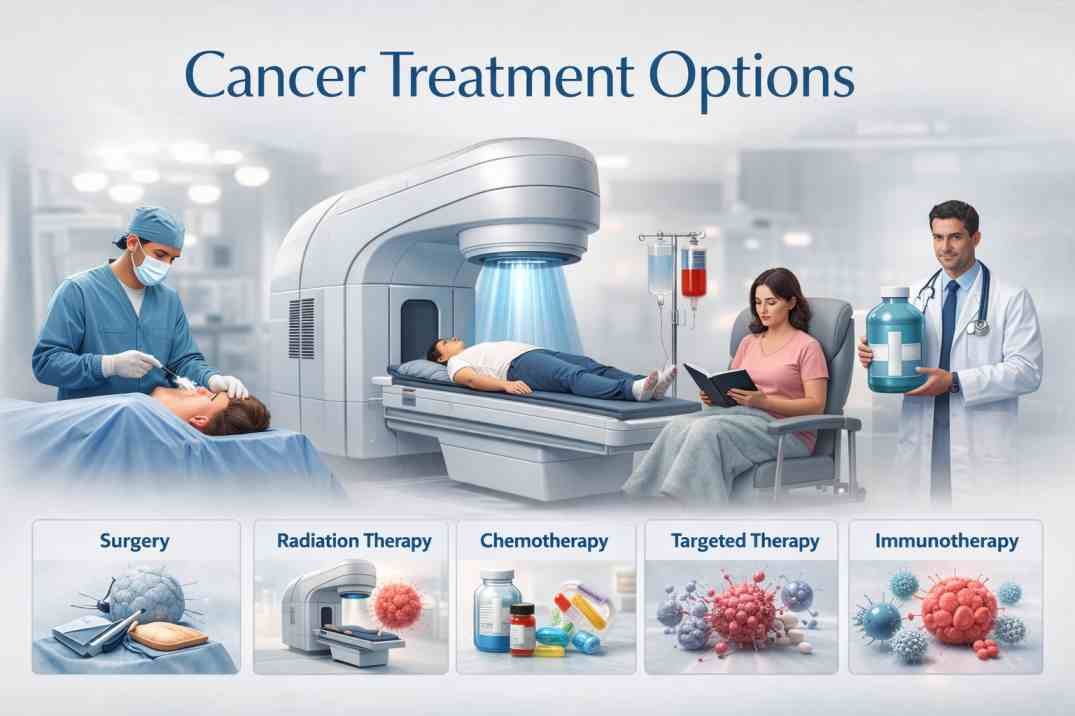
Types of Cancer Treatments and Medications: Comprehensive Overview of How They Work
Chromogranin A (CgA): is a tumor marker associated with neuroendocrine tumors (NETs).
Chromogranin A is a marker used for neuroendocrine tumors, including carcinoid tumors and pancreatic neuroendocrine tumors. It can also be elevated in non-cancerous conditions such as stress and hypertension. Chromogranin A is used to monitor treatment and detect recurrence.
The Chromogranin A (CgA) is a protein that is produced by neuroendocrine cells in various organs throughout the body, including the gastrointestinal tract, pancreas, and lungs. It is a member of the granin family of proteins, which play important roles in regulating the secretion and storage of hormones and other signaling molecules.
Measuring CgA levels in the blood can be used as a diagnostic tool for certain types of neuroendocrine tumors, including carcinoid tumors, gastrinomas, and insulinomas. In these types of tumors, CgA is often produced by the tumor cells and can be detected in the blood.
The cancers that CgA is used to detect and monitor include:
- Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): CgA is most commonly used as a tumor marker for gastroenteropancreatic neuroendocrine tumors, which include tumors arising from the digestive system (stomach, intestines, pancreas, etc.). GEP-NETs can be functional, producing hormones, or non-functional.
- Pulmonary Neuroendocrine Tumors: CgA can also be elevated in certain types of lung tumors with neuroendocrine features, such as carcinoid tumors.
- Other Neuroendocrine Tumors: CgA levels may also be elevated in other types of neuroendocrine tumors, such as pheochromocytomas and paragangliomas.
CgA levels can also be used to monitor the progression of these tumors and to assess the response to treatment. However, it is important to note that CgA levels can also be elevated in other medical conditions, such as chronic kidney disease, liver disease, and certain medications, so it is not a specific marker for neuroendocrine tumors and should be used in conjunction with other diagnostic tests and clinical assessments.
Cancer antigen 72-4 (CA 72-4): is a tumor marker associated with certain types of gastrointestinal cancers, particularly stomach (gastric) cancer.
CA 72-4 is a marker used for gastrointestinal cancers, including stomach and colon cancers. It can also be elevated in non-cancerous conditions such as pancreatitis and liver disease. CA 72-4 is used to monitor treatment and detect recurrence.
The Cancer antigen 72-4 (CA 72-4) is a protein that is sometimes found in higher than normal levels in the blood of patients with certain types of cancer, particularly gastrointestinal and ovarian cancers. CA 72-4 is a member of the glycoprotein family of tumor markers, which are substances that can be detected in the blood or other bodily fluids and are often produced by cancer cells.
Measuring CA 72-4 levels in the blood can be used as a diagnostic tool for certain types of cancer, particularly those involving the stomach, pancreas, or ovaries. It can also be used to monitor the progression of these cancers and to assess the response to treatment.
The cancers that CA 72-4 is used to detect and monitor include:
- Stomach (Gastric) Cancer: CA 72-4 is most commonly used as a tumor marker for stomach cancer. Elevated CA 72-4 levels can be observed in some individuals with gastric cancer, particularly in advanced stages of the disease.
- Ovarian Cancer: In addition to stomach cancer, CA 72-4 may also be elevated in some cases of ovarian cancer.
However, it is important to note that CA 72-4 levels can also be elevated in other medical conditions, such as liver disease, pancreatitis, and certain medications, so it is not a specific marker for cancer and should be used in conjunction with other diagnostic tests and clinical assessments.
Squamous cell carcinoma antigen (SCC): is a tumor marker associated with squamous cell carcinomas, which are a type of cancer that can arise from squamous cells. Squamous cells are flat, thin cells that are found in the skin, the lining of various organs, and the respiratory and digestive tracts.
SCC is a marker used for squamous cell carcinoma of the head and neck, cervix, and lung. It can also be elevated in non-cancerous conditions such as psoriasis and chronic obstructive pulmonary disease (COPD). SCC is used to monitor treatment and detect recurrence.
The Squamous cell carcinoma antigen (SCC) is a protein that is found in higher than normal levels in the blood of some patients with squamous cell carcinomas, which are a type of skin cancer that can also occur in other parts of the body, such as the lungs, cervix, and head and neck.
Measuring SCC levels in the blood can be used as a diagnostic tool for squamous cell carcinomas and as a way to monitor the effectiveness of treatment. SCC levels can also be used to predict the risk of recurrence and to monitor for any signs of cancer recurrence after treatment.
The cancers that SCC is used to detect and monitor include:
- Squamous Cell Carcinoma of the Skin: SCC is most commonly used as a tumor marker for squamous cell carcinoma of the skin, a type of skin cancer that can develop on sun-exposed areas of the body. SCC of the skin is one of the most common types of skin cancer.
- Cervical Squamous Cell Carcinoma: SCC testing may also be used in the management of cervical cancer, particularly squamous cell carcinoma of the cervix.
- Head and Neck Squamous Cell Carcinomas: SCC of the head and neck region, which includes cancers of the mouth, throat, larynx, and nasal cavity, may also be associated with elevated SCC levels.
However, it is important to note that SCC levels can also be elevated in other medical conditions, such as psoriasis, eczema, and certain infections, so it is not a specific marker for squamous cell carcinomas and should be used in conjunction with other diagnostic tests and clinical assessments.
Neuron-specific enolase (NSE): is a tumor marker associated with certain types of neuroendocrine tumors, particularly those that originate from neuroendocrine cells.
NSE is a marker used for neuroendocrine tumors, small cell lung cancer, and some other types of cancer. It can also be elevated in non-cancerous conditions such as stroke and neurodegenerative disorders. NSE is used to monitor treatment and detect recurrence.
Neuron-specific enolase (NSE) is a type of protein that is produced by neurons and neuroendocrine cells in the body, particularly in the brain and central nervous system. It is an important marker for the diagnosis and monitoring of certain types of neuroendocrine tumors, including small cell lung cancer and neuroblastoma.
Measuring NSE levels in the blood or cerebrospinal fluid (CSF) can be used as a diagnostic tool for these types of tumors and to monitor their progression and response to treatment. NSE levels can also be used to predict the risk of recurrence and to monitor for any signs of cancer recurrence after treatment.
The cancers that NSE is used to detect and monitor include:
- Neuroendocrine Tumors: NSE is most commonly used as a tumor marker for neuroendocrine tumors, which can arise in various organs, including the lungs, gastrointestinal tract, pancreas, and other parts of the body. Neuroendocrine tumors are a diverse group of cancers that produce hormones and other substances, and they can have varying degrees of aggressiveness.
- Small Cell Lung Cancer (SCLC): NSE is particularly useful in the diagnosis and monitoring of small cell lung cancer, a type of lung cancer that is often associated with neuroendocrine features.
However, it is important to note that NSE levels can also be elevated in other medical conditions, such as stroke, brain injury, and certain infections, so it is not a specific marker for neuroendocrine tumors and should be used in conjunction with other diagnostic tests and clinical assessments.
Gastrointestinal stromal tumor-1 (GIST-1): is not a commonly recognized tumor marker in the medical literature. However, there might be some confusion regarding the terminology.
Gastrointestinal stromal tumors (GISTs) are a specific type of tumor that originates from the interstitial cells of Cajal, which are specialized cells in the gastrointestinal tract that regulate its movements. GISTs are typically characterized by the presence of mutations in the KIT or PDGFRA genes.
GIST-1 is a marker used to detect gastrointestinal stromal tumors (GISTs), which are rare tumors that arise in the digestive tract. GISTs are commonly found in the stomach or small intestine, but can also occur in other parts of the GI tract.
GIST-1 is a protein found on the surface of GIST cells, and is detected using an immunohistochemical (IHC) assay. The IHC test involves staining a tissue sample with an antibody that is specific for GIST-1, and then examining the sample under a microscope to see if staining is present. If staining is present, it indicates that the tumor is positive for GIST-1.
GIST-1 detection is important because it aids in the diagnosis of GIST, which can be difficult to diagnose on the basis of symptoms alone. GIST is often resistant to chemotherapy and radiation therapy, but can be treated with targeted therapy drugs that specifically target the proteins that cause tumors to grow. GIST-1 testing can also help doctors determine the best treatment approach for patients with GIST.
Fibroblast growth factor 23 (FGF23): is not typically considered a tumor marker for cancer detection. FGF23 is a protein that plays a critical role in regulating phosphate and vitamin D metabolism in the body.
FGF23 is a marker used to detect mesenchymal phosphate tumors (PMT), which are rare tumors that cause a rare bone disease called tumor-induced osteomalacia.
It is primarily associated with certain rare genetic disorders, including hereditary hypophosphatemic rickets and autosomal dominant hypophosphatemic rickets.
FGF23 is not routinely used as a tumor marker in the diagnosis or monitoring of cancer. Instead, it is mainly studied in the context of metabolic bone disorders and kidney-related conditions.
Fibroblast growth factor 23 (FGF23) is a tumor marker used to detect mesenchymal phosphate tumors (PMT), which are rare tumors that cause a rare bone disease called tumor-induced osteomalacia. PMT is a slow-growing tumor that is usually found in the bones or soft tissues of the body.
FGF23 is a hormone produced by osteocytes and osteoblasts in bone, and helps regulate phosphate and vitamin D metabolism in the body. In PMT, FGF23 production is very high, which can lead to wasted phosphate and low blood levels of vitamin D. This can cause a variety of symptoms, including muscle weakness, bone pain, and fractures.
Testing for FGF23 is done using a blood test, which measures levels of FGF23 in the blood. Elevated FGF23 levels may indicate PMT, although other conditions can cause elevated FGF23 levels, such as chronic kidney disease and hypophosphatemic rickets.
FGF23 detection is important because it can help diagnose PMT and differentiate it from other conditions that cause similar symptoms. Surgical removal of the tumor is usually the treatment of choice for PMT, and early detection and treatment can help prevent complications and improve outcomes.
Isocitrate dehydrogenase 1 (IDH1) and Isocitrate dehydrogenase 2 (IDH2) mutations: are genetic mutations that have been identified in certain types of cancers. While they are not traditional tumor markers used for cancer detection or screening, they are important molecular markers that can aid in the diagnosis, prognosis, and treatment of specific cancers. associated with certain types of brain tumors, including gliomas.
They are rare tumor markers associated with certain types of brain tumors, including gliomas.
Isocitrate dehydrogenase 1 (IDH1) and Isocitrate dehydrogenase 2 (IDH2) are enzymes that play a role in cell metabolism, especially in the citric acid cycle. Mutations in this gene have been found in various types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
The most common mutations in IDH1 and IDH2 result in a single amino acid substitution, which causes the enzyme to produce an abnormal metabolite, 2-hydroxyglutarate (2-HG). 2-HG accumulation has been shown to interfere with normal cellular processes and contribute to cancer development and progression.
Testing for IDH1 and IDH2 mutations is important for the diagnosis and management of certain types of cancer. In glioma and AML, for example, the presence of an IDH mutation is a diagnostic criterion and can help determine prognosis and treatment options. In some cases, targeted therapies have been developed that specifically target mutant IDH enzymes and have shown promise in clinical trials.
The cancers associated with IDH1 and IDH2 mutations include:
- Gliomas: IDH1 and IDH2 mutations are commonly found in various types of brain tumors, including gliomas, which are tumors that originate from glial cells in the brain.
- Acute Myeloid Leukemia (AML): IDH1 and IDH2 mutations are also seen in a subset of patients with AML, a type of blood cancer that affects myeloid cells in the bone marrow.
- Chondrosarcomas: These mutations can also be found in chondrosarcomas, which are malignant bone tumors.
In addition to its role as a diagnostic and prognostic marker, IDH mutations are also being studied as potential therapeutic targets for cancer treatment. Researchers are looking for ways to block 2-HG production or target the effects of other mutations downstream, which could lead to new treatments for this type of cancer.
It’s important to note that the use of tumor markers in cancer diagnosis and management is constantly evolving. The interpretation of tumor marker results should always be done in conjunction with other diagnostic tests, a person’s overall medical history, and any other relevant information.
Information: Cleverly Smart is not a substitute for a doctor. Always consult a doctor to treat your health condition.
Sources: PinterPandai, Medline Plus, Cancer.Net, National Center for Biotechnology Information: NCBI (U.S. National Library of Medicine), Science Direct, National Cancer Institute
Photo credit: Belova59 via Pixabay