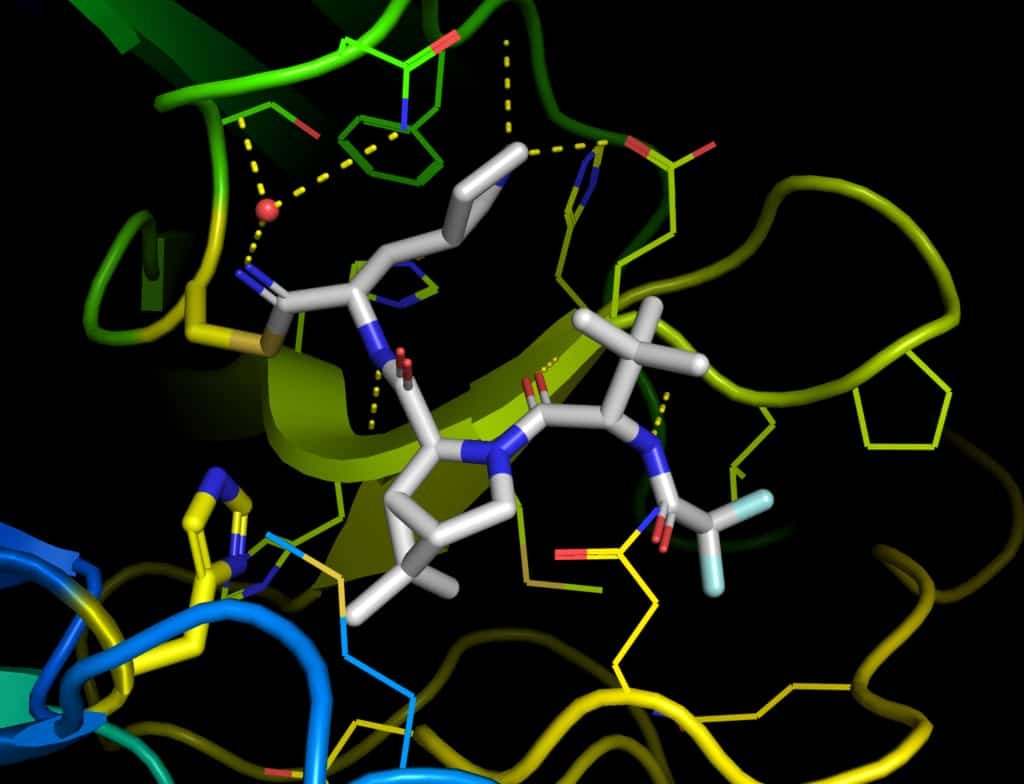
Paxlovid
A new, more manageable and effective treatment against Covid variants, in particular Omicron, will be available in the first quarter of 2022 (mid-February 2022). This is Paxlovid, an oral treatment specially designed by Pfizer to be administered orally at the first sign of Covid infection. The European Medicines Agency (EMA) informed on November 19 that it was starting its review “to help national authorities decide on its early use for Covid-19, for example in emergency situations, before the marketing authorization”.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) will review data from a study comparing the effect of Paxlovid to that of a dummy treatment (placebo) in outpatients with mild to moderate Covid who presented with a high risk of progressing to serious illness. Pfizer also applied for emergency use authorization on November 16, 2021 from the Food and Drug Administration (FDA), the US Food and Drug Administration. How does this pill work? Its effectiveness against Covid? Its side effects? Knowledge to date.
Paxlovid approved on December 22, 2021
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
Paxlovid is available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset. Paxlovid is not authorized for use for longer than five consecutive days.
What is Paxlovid?
The American pharmaceutical company Pfizer has developed an antiviral treatment, marketed under the name Paxlovid. If it obtains the marketing authorizations, this pill, administered orally, could be taken at home in the first days after the appearance of symptoms in the event of infection with Covid-19. Paxlovid is a treatment similar to Molnupiravir, an antiviral drug developed by the US pharmaceutical company Merck. Already authorized since the beginning of November 2021 in the United Kingdom, this antiviral has been the subject of an emergency authorization request in the United States, which must be examined on November 30, 2021 by the FDA. The French government would have ordered 50,000 doses, indicated the Minister of Health Olivier Véran at the end of October, pending validation from the European Medicines Agency.
How effective is it against Covid and against Omicron?
Paxlovid would be able to reduce the risk of hospitalization or death by 89%.
Paxlovid would be particularly effective against the variants of Covid – and therefore Delta and Omicron – because this treatment is directed against the protease of the virus which is not very modified (protease inhibitor), explains the Scientific Council in an opinion of December 16, 2021.
At the time of ” a randomized, double-blind, phase 2/3 interim analysis conducted on more than 1,800 outpatients in July 2021, all with at least one risk factor for developing a severe form of the disease, Pfizer showed that Paxlovid was able to reduce the risk of hospitalization or death by 89% compared to the group that received a placebo, reports the laboratory in a press release dated November 5, 2021. Each patient received the treatment or oral placebo every 12 hours during 5 days.
For patients who received Paxlovid treatment or placebo within 3 days of symptom onset:
Hospitalizations: 0.8% (or 3 of 389) of patients who received Paxlovid were hospitalized until day 28 compared to 7% of patients (or 27 of 385) who received placebo.
Deaths: No deaths were reported in patients who received Paxlovid compared to 7 in patients who received placebo.
For patients who received Paxlovid treatment or placebo within 5 days of symptom onset:
Hospitalizations: 1% (or 6 out of 607) of patients who received Paxlovid were hospitalized until day 28 versus 6.7% of patients (or 41 out of 612) who received placebo.
Deaths: No deaths were reported in patients who received Paxlovid compared to 10 in patients who received placebo.
“These data suggest that our oral antiviral candidate, if approved or cleared by regulatory authorities, has the potential to save patient lives, reduce the severity of COVID-19 infections, and eliminate up to 9 in 10 hospitalizations, said Albert Bourla, Chairman and CEO of Pfizer. Subject to approval or clearance, it could be prescribed more widely as a home treatment to help reduce the severity of illness, and deaths, as well as the likelihood of infection, after exposure, in adults”.
What is the composition of Paxlovid?
Paxlovid is made up of two molecules. The first PF-07321332 is the active agent which prevents the virus protein from replicating. The second is ritonavir, a protease inhibitor, normally used for the treatment of HIV infections. “HIV is not related to coronaviruses and the replication of its genetic material takes place by a different mechanism.
But it too produces a poly-protein which must be cut 9 times by a protease to form the new viral particles”, explains an article published in the scientific journal Medicine Science in June 2020.
Mode of action: how does Paxlovid work?
Paxlovid is said to prevent the coronavirus protease from replicating.
A protease is a virus-specific enzyme that acts on viral proteins to promote virus replication.
To stop the replication of a virus, it is therefore necessary to reduce or prevent the protease from acting. Coronaviruses have two proteases, one of which is called SARS-CoV-2, 3CL. It is on this protease that the oral Paxlovid treatment works.
What are the side effects?
Of the patients who participated in the study and received Paxlovid, 1.7% experienced serious adverse events and had to stop treatment. The active substance in this medicine, ritonavir, can indeed cause side effects such as:
- diarrhea,
- nausea and vomiting
- abdominal pain
- throat irritation or cough
- headaches and dizziness
- rashes and itching
- disturbances in the metabolism of sugars and fats with an abnormal redistribution of fats observed after several
- months of treatment (called lipodystrophy)
- toxicity to the liver (increased transaminases) and the pancreas.
What is the price ?
The price of the Paxlovid pill is not yet known. Nonetheless, Pfizer announced in its statement tiered pricing based on the income level of each country to ensure equitable access for all and an affordable price. In other words, high and upper middle income countries will pay more than low income countries.
Information: Cleverly Smart is not a substitute for a doctor. Always consult a doctor to treat your health condition.
Sources:
– PFIZER’S NOVEL COVID-19 ORAL ANTIVIRAL TREATMENT CANDIDATE REDUCED RISK OF HOSPITALIZATION OR DEATH BY 89% IN INTERIM ANALYSIS OF PHASE 2/3 EPIC-HR STUDY, November 5, 2021.
– Targeting the major SARS-CoV-2 protease to manufacture an effective drug against this coronavirus, Medecine Science, June 2020.
– PinterPandai, Pfizer, CNN, Washington Post, European Medicines Agency, The Guardian, U.S. Food and Drug Administration
Photo credit: Vcpmartin / Wikimedia Commons (CC BY-SA 4.0)
Photo description:: the crystal structure1 of the SARS-CoV-2 protease inhibited by PF-07321332.