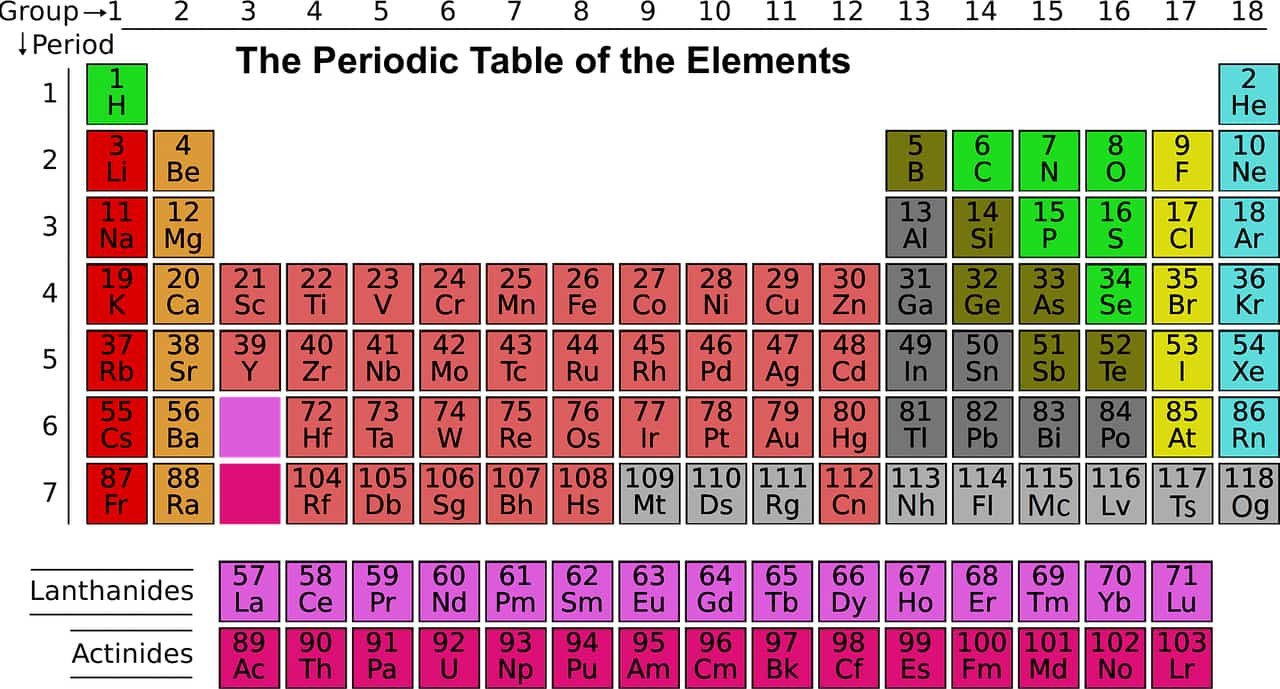
Period of the Periodic Table from 1 to 7
In the periodic table of the elements, a period of the periodic table is a row of the table from 1 to 7, they are the chemical elements of the same period have the same number of electron layers. Seven periods contain the elements observed to date, and a hypothetical eighth period has been described.
The organization of the table in rows called periods and columns called groups reflects the periodicity of the physicochemical properties of the elements as the atomic number increases. Elements of the same group have similar properties despite having different atomic mass, while adjacent elements in the same period have similar mass but different chemical properties.
The atomic number (Z) represents, in chemistry and physics, the number of protons in an atom. The latter can be schematized, as a first approach, by a compact agglomeration (atomic nucleus) of protons (p +) and neutrons (n), around which electrons (e−) circulate.
Period of the Periodic Table 1
| Group | 1/17 | 2/18 |
|---|---|---|
| Z symbole | 1 H | 2 He |
Period 1 only includes hydrogen and helium. Although they both belong to the s block, they do not have the same properties as the other elements of this block. Hydrogen easily gains an electron and is not a metal under normal conditions of temperature and pressure. Helium exhibits the chemical properties of a noble gas and, for this reason, is attached to group 18 of the periodic table.
Period of the Periodic Table 2
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|
| Z symbol | 3 Li | 4 Be | 5 B | 6 C | 7 N | 8 O | 9 F | 10 Ne |
The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine and neon. This situation can be explained by the modern theory of the structure of the atom. In a quantum mechanical explanation of the structure of the atom, this period is related to the filling of the 2s and 2p orbitals. Period 2 elements adhere to the octet rule, which means they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can hold is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All the elements in this period can form diatomic molecules except beryllium and neon.
Period of the Periodic Table 3
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|
| Z symbol | 11 Na | 12 Mg | 13 Al | 14 Si | 15 P | 16 S | 17 Cl | 18 Ar |
The third period consists of eight elements: sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. It should be noted that there is already a 3d subshell, but it doesn’t fill up until period 4, this kind of thing giving the periodic table a characteristic shape of “two rows at a time”. All Period 3 elements occur in nature and contain at least one stable isotope.
Period of the Periodic Table 4
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z symbol | 19 K | 20 Ca | 21 Sc | 22 Ti | 23 V | 24 Cr | 25 Mn | 26 Fe | 27 Co | 28 Ni | 29 Cu | 30 Zn | 31 Ga | 32 Ge | 33 As | 34 Se | 35 Br | 36 Kr |
The fourth period of the periodic table of the elements contains all chemical elements that have exactly four electron shells in the atom . The innermost (first) electron shell is fully occupied and has two electrons . The second electron shell is also fully occupied and has eight electrons. The third electron shell has a minimum of eight and a maximum of 18 electrons. The outermost (fourth) electron shell, also known as the valence shell , can hold between one and eight electrons. Thus there are a total of 18 chemical elements in the fourth period.
Period of the Periodic Table 5
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z symbol | 37 Rb | 38 Sr | 39 Y | 40 Zr | 41 Nb | 42 Mo | 43 Tc | 44 Ru | 45 Rh | 46 Pd | 47 Ag | 48 Cd | 49 In | 50 Sn | 51 Sb | 52 Te | 53 I | 54 Xe |
The fifth period of the periodic table of the elements contains all chemical elements that have exactly five electron shells in the atom . The innermost (first) electron shell is fully occupied and has two electrons . The second electron shell with 8 electrons and the third electron shell with 18 electrons are also fully occupied. The fourth electron shell has at least eight electrons and can hold a maximum of 18 electrons. The outermost (fifth) electron shell, also known as the valence shell , can hold between one and eight electrons. Thus there are a total of 18 chemical elements in the fifth period.
Period of the Periodic Table 6
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z symbol | 55 Cs | 56 Ba | 57 La | 58 Ce | 59 Pr | 60 Nd | 61 Pm | 62 Sm | 63 Eu | 64 Gd | 65 Tb | 66 Dy | 67 Ho | 68 Er | 69 Tm | 70 Yb | 71 Lu | 72 Hf | 73 Ta | 74 W | 75 Re | 76 Os | 77 Ir | 78 Pt | 79 Au | 80 Hg | 81 Tl | 82 Pb | 83 Bi | 84 Po | 85 At | 86 Rn |
The sixth period of the periodic table of the elements contains all chemical elements that have exactly six electron shells in the atom . The innermost (first) electron shell is fully occupied and has two electrons . The second electron shell with eight electrons and the third electron shell with 18 electrons are also fully occupied. The fourth electron shell has at least 18 electrons and can hold a maximum of 32 electrons. The fifth electron shell has at least 8 electrons and can hold a maximum of 18 electrons. The outermost (sixth) electron shell, also known as the valence shellcalled, can hold between one and eight electrons. Thus there are a total of 32 chemical elements in the sixth period. The lanthanides represent a special group within this period.
Period of the Periodic Table 7
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z symbol | 87 Fr | 88 Ra | 89 Ac | 90 Th | 91 Pa | 92 U | 93 Np | 94 Pu | 95 Am | 96 Cm | 97 Bk | 98 Cf | 99 Es | 100 Fm | 101 Md | 102 No | 103 Lr | 104 Rf | 105 Db | 106 Sg | 107 Bh | 108 Hs | 109 Mt | 110 Ds | 111 Rg | 112 Cn | 113 Nh | 114 Fl | 115 Mc | 116 Lv | 117 Ts | 118 Og |
All of the elements of period 7 are radioactive. This period contains actinides, which contain the heaviest naturally occurring element, californium; the next element must be synthesized artificially. Although one of these (einsteinium) is now available in macroscopic amounts, most of the others are very rare, only made in micrograms or less. Lastly, the element transactinides have only been identified in the laboratory as stacks of a few atoms at a time.
Although the rarity of many of these elements suggests that the experimental results are not very broad, the definition of periodic trends and their groups is less favorable than for other periods. While francium and radium exhibit characteristic properties of their respective groups, actinides display much greater variation in behavior and oxidation state than do lanthanides. This peculiarity is due to a variety of factors, including the large degree of spin-orbit coupling (English: spin-orbit coupling) and relativistic effects, mainly due to the very high positive electric charges in their massive atomic nuclei.
Period of the Periodic Table 8 and 9
Period 8 of the periodic table is the eighth row, or period, of the extended versions of the Periodic Table of the Elements. It contains hypothetical chemical elements: as of June 2017, none of these elements had been observed, although various attempts to synthesize them have taken place since the beginning of the 21st century. These elements are designated according to the systematic name of the IUPAC: the first two of them, for example, are thus called respectively ununennium and unbinilium, which corresponds to the numbers 1-1-9 and 1-2-0 of their respective atomic number; in the literature, the systematic designation is never used, and an element with atomic number Z is simply referred to as “element Z” – ununennium and unbinilium are thus always referred to as “element 119” and “element 120”.
The study of the elements of period 8 is more a matter of nuclear physics, or even particle physics, than chemistry, because the first step is to achieve their synthesis and detection. If we managed to produce sufficient quantities to be able to study its chemistry, these elements would certainly exhibit different behaviors from those of previous periods due to an electronic configuration altered by quantum and relativistic effects becoming sensitive to these energy levels. , such as quantum electrodynamics, or spin-orbit coupling, which divides the peripheral sub-layers by recomposing the distribution of energy levels to form new apparent sub-layers unrelated to the periodicity observed for the elements of lower atomic number.
Period 9 of the periodic table is the ninth line, or period, of some extended versions of the periodic table of the elements. It contains hypothetical chemical elements the study of which is pure theory and does not cover any experimental reality, insofar as the detection of the first element of the 8th period, element 119, is already at the extreme limit of resolution of infrastructures existing in December 2016: the experimental limit of the periodic table is generally located around Z = 130.
Periodic Table of Elements | Complete List of Chemical Elements by Group, Name, Symbol, Color and Type